
The Top 10 FDA 483 Observations and Strategies for Compliance
FDA Form 483 observations spotlight critical compliance gaps in pharmaceutical manufacturing. This blog explores the top 10 most common findings, from inadequate procedures and poor record-keeping to insufficient training, equipment qualification failures, and data integrity lapses. Through practical insights and case studies, it guides professionals in understanding root causes, implementing corrective actions, and fostering a proactive culture of compliance. Strengthen your readiness, avoid enforcement actions, and drive continuous improvement to uphold safety, quality, and regulatory excellence.

Managing Spreadsheets in GMP Manufacturing: A Comprehensive Quality Professional’s Guide
Managing spreadsheets in pharmaceutical manufacturing demands rigorous compliance with GMP regulations and 21 CFR Part 11 requirements. Quality professionals must establish controlled issuance systems, implement validation protocols using GAMP frameworks, and ensure data integrity through ALCOA+ principles. Critical elements include audit trails, electronic signatures, periodic reviews, and change control procedures. Without proper governance, spreadsheets become regulatory vulnerabilities. However, systematic approaches transform these flexible tools into compliant assets that support product quality while meeting FDA scrutiny and patient safety obligations.

7 Steps to Develop Lean-Compliant SOPs for Regulatory Success
In today's compliance-driven environment, developing lean and effective SOPs is critical for regulatory success. This article presents a seven-step framework integrating Lean principles with cGMP requirements to streamline documentation, enhance clarity, and build a culture of continuous improvement. From engaging subject matter experts to leveraging automation and visual aids, each step empowers organizations to reduce waste, improve quality, and maintain FDA readiness. Practical strategies promote sustainable compliance and operational excellence across highly regulated industries.

Strengthening GMP Processes Through Strategic Supplier Agreements
This blog explores how strategic supplier agreements can reinforce GMP compliance and enhance supply chain resilience in pharmaceutical manufacturing. It emphasizes the importance of robust quality agreements, supplier qualification, risk management, digital transformation, and continuous improvement. By fostering transparent, quality-centric partnerships, companies can better manage regulatory obligations, safeguard product integrity, and maintain global market access. The post offers practical insights into building a quality-driven supply network that supports operational excellence and sustained regulatory compliance.
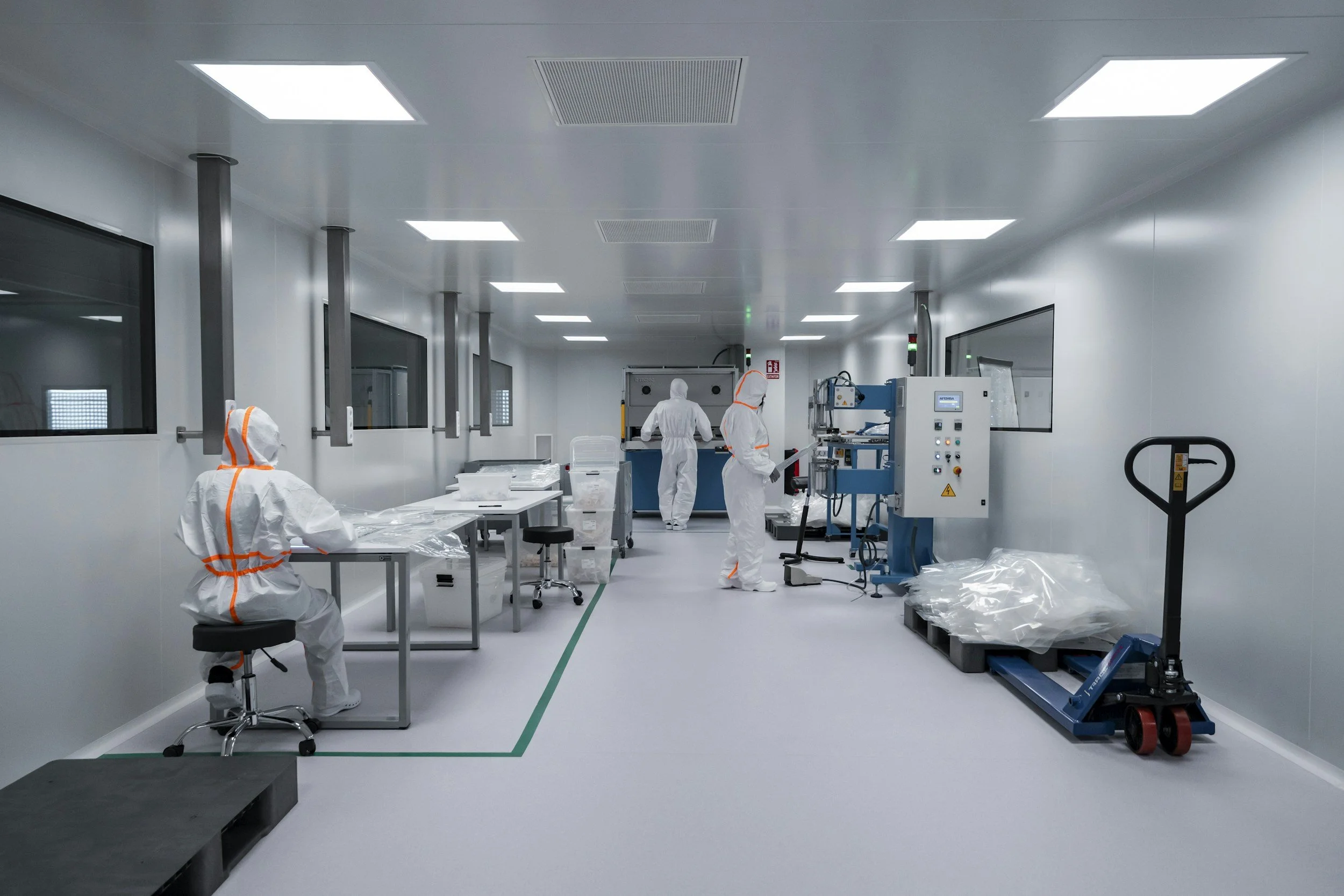
From Protocol to Practice: Implementing Effective Cleaning Validation
Cleaning validation is essential in pharmaceutical manufacturing to ensure equipment is free from contaminants that could compromise product quality and patient safety. This blog explores the development of risk-based cleaning protocols, verification methods such as visual inspection and ATP testing, and emerging trends like digitalization and CIP systems. Through regulatory insights and a real-world case study, it highlights how companies can bridge theoretical protocols with practical applications to achieve compliance, prevent cross-contamination, and maintain the highest cleanliness standards.

How to Design GMP Training Programs That Withstand Inspections
A robust GMP training program is essential for pharmaceutical companies to ensure inspection readiness and maintain compliance. This blog explores foundational elements like Good Documentation Practices (GDP), SOP development, and digital integration, highlighting strategies such as training needs assessments, mock inspections, and internal audits. By leveraging flexible learning formats and emphasizing documentation accuracy, companies can foster a culture of continuous improvement, enhance employee competence, and reduce compliance risks—ultimately safeguarding product quality and regulatory standing.

The Role of Psychological Safety in GMP Manufacturing: From Compliance to Commitment
In GMP manufacturing, psychological safety transforms compliance into commitment by empowering employees to speak up, learn, and innovate without fear. This blog explores how psychological safety supports knowledge retention, mitigates learning decay, and fosters a culture of continuous improvement. Strategies such as role-specific training, job aids, mentoring, and scenario-based learning enhance competence and quality. With strong leadership commitment, psychological safety becomes the foundation for sustainable GMP excellence, promoting both operational precision and a resilient, quality-first workforce.

Harnessing Gemba Walks to Elevate GMP Standards on the Shop Floor
Gemba Walks are transforming GMP compliance by enabling real-time observation, employee engagement, and continuous improvement on the manufacturing floor. This blog explores how structured, respectful Gemba Walks—supported by checklists, digital tools, and Lean principles—help identify inefficiencies, promote safety, and reinforce quality standards. By aligning leadership insight with shop-floor realities, Gemba Walks drive operational excellence, enhance morale, and ensure GMP adherence. They are a foundational practice for modern, proactive, and compliant pharmaceutical manufacturing environments.

Leveraging Quality Metrics for Superior GMP Performance Monitoring
In the pharmaceutical industry, quality metrics are vital tools for ensuring GMP compliance, enhancing operational efficiency, and promoting continuous improvement. This blog explores the strategic integration of quality metrics within Quality Management Systems, regulatory expectations from bodies like the FDA and EMA, and the benefits of electronic QMS adoption. It emphasizes the role of KPIs, data analytics, and Quality Management Reviews in safeguarding product integrity, driving innovation, and maintaining regulatory alignment across the product lifecycle.

Data Integrity and AI Integration: Key Considerations for Compliance in GMP Pharmaceutical Manufacturing
As AI reshapes pharmaceutical manufacturing, maintaining data integrity becomes more critical than ever. This blog explores how companies can harness AI’s power—improving efficiency, predictive capabilities, and compliance—while upholding ALCOA+ principles and meeting evolving FDA and EMA regulatory expectations. It outlines key benefits, challenges, and strategies for successful AI adoption, emphasizing robust data management, explainability, and cybersecurity. By aligning AI systems with GMP standards, manufacturers can drive innovation without compromising product quality or regulatory trust.

The Intersection of Quality Culture and Innovation in Manufacturing
In an era of rapid transformation, this blog explores how a thriving quality culture can fuel—not hinder—innovation in GMP manufacturing. It reveals how visionary leadership, advanced technologies, and empowered employees can seamlessly merge compliance with creativity. From FDA-aligned standards to digital QMS tools, the piece illustrates how companies can turn regulatory rigor into a launchpad for excellence. With real-world insights and forward-thinking strategies, this post is a blueprint for building bold, quality-driven innovation in pharma manufacturing.

The History of Good Manufacturing Practices (GMP): From Tragedy to Trust
From tragic public health events to global regulatory harmonization, this blog traces the evolution of Good Manufacturing Practices (GMP) from their early roots to modern-day compliance frameworks. It explores pivotal moments like the Elixir Sulfanilamide disaster, the thalidomide crisis, and the rise of risk-based inspection models. With a forward-looking lens on data integrity, digital transformation, and AI integration, the post underscores how GMP continues to adapt—protecting patients and elevating pharmaceutical quality standards worldwide.

The Impact of AI on GMP Operations: Promise, Pitfalls, and the Path Forward
Artificial intelligence is reshaping GMP operations by accelerating deviation management, enhancing batch record review, and supporting inspection readiness. But alongside these gains come serious challenges, including data integrity risks, validation complexity, and regulatory uncertainty. This blog explores the transformative potential of AI—especially large language models—in pharmaceutical manufacturing, while offering practical guidance on mitigating pitfalls. Learn how to harness AI safely within GMP environments without compromising compliance, transparency, or the foundational principles of quality and accountability.

The Critical Role of Executive Leadership in Shaping GMP Culture
Strong GMP culture starts at the top. Executive leadership sets the tone for quality, influencing behaviors, decisions, and compliance across the organization. This blog explores how visible engagement, strategic communication, and ethical accountability drive sustainable GMP success—and what happens when leadership fails. Drawing on real-world cases like Ranbaxy and NECC, it underscores why leaders must champion GMP not as a checklist, but as a core value essential to product integrity and patient safety.

Welcome to the Auria GMP Blog: Your Trusted Resource for Quality and Compliance
Welcome to the Auria GMP Blog—your trusted source for expert insights on pharmaceutical quality and compliance. From inspection readiness to data integrity and AI integration, we explore real-world challenges and solutions to help life sciences professionals build stronger systems and drive sustainable GMP excellence across their organizations.